西安秋然试验室张航冷聚变实验2021年总结
2022-01-05 10:24:37 来源:冷聚变世界 评论:0 点击:
日本高桥教授在23届国际凝聚态核科学会议上发布了“铜镍锆合金在氢气中的多余热量的实验”,每公斤合金可以产生200瓦的多余热量,秋然实验室对该实验进行了复制,观察到了每公斤合金最高24瓦的多余热量。
锆合金在氢气中的多余热量
Excess heat of zirconium alloy in hydrogen
秋然实验室
Qiuran Laboratory
张航 email:715469127@qq.com
Zhang hang email: 715469127@qq.com
Qiuran Laboratory
张航 email:715469127@qq.com
Zhang hang email: 715469127@qq.com
摘要
abstract
日本高桥教授在23届国际凝聚态核科学会议上发布了“铜镍锆合金在氢气中的多余热量的实验”,每公斤合金可以产生200瓦的多余热量,秋然实验室对该实验进行了复制,观察到了每公斤合金最高24瓦的多余热量。
Professor Takahashi of Japan released the "experiment of excess heat of copper nickel zirconium alloy in hydrogen" at the 23rd International condensed matter nuclear science conference. 200 watts of excess heat can be generated per kilogram of alloy. Akiran laboratory copied the experiment and observed the maximum excess heat of 24 watts per kilogram of alloy.
关键词
key word
铜镍锆合金、氢气、量热计、多余热量
Copper nickel zirconium alloy, hydrogen, calorimeter, excess heat
1、 高桥教授实验介绍
1、 Introduction to Professor Takahashi's experiment
日本的实验用铜镍锆合金在氢气中加热,铜镍锆合金摩尔比是1:7:14,观察多余热量,经过多次氧化后多余热量明显升高,可以维持数周,最高多余热量200瓦/公斤,实验数据如下图
The experimental copper nickel zirconium alloy in Japan is heated in hydrogen. The molar ratio of copper nickel zirconium alloy is 1:7:14. Observe the excess heat. After multiple oxidation, the excess heat increases significantly and can be maintained for several weeks. The maximum excess heat is 200 W / kg. The experimental data are shown in the figure below。
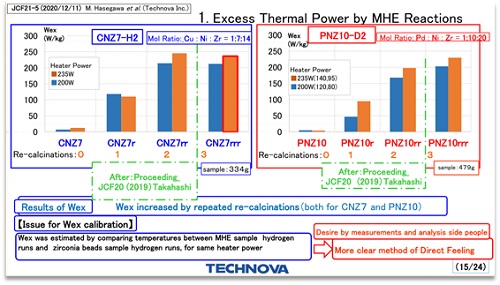
图1 高桥教授实验数据图
Fig. 1 experimental data of Professor Takahashi
2、实验步骤
2. Experimental steps
2.1 材料制备
2.1 material preparation
2.1.1 将铜镍锆粉末按照摩尔比1:7:14混合,放入球磨罐,添加水作为分散剂,在球磨机上球磨,进行金属合金化,球磨时间100小时。
2.1. 1. Mix the copper nickel zirconium powder according to the molar ratio of 1:7:14, put it into the ball milling tank, add water as dispersant, ball mill it on the ball mill for metal alloying, and the ball milling time is 100 hours.
2.1.2 取出铜镍锆合金粉末,干燥,称量250克,加入250克二氧化锆纳米粉末,放入球磨罐,添加水作为分散剂,在球磨机上进行球磨混合,混合时间100小时。
2.1. 2 take out the copper nickel zirconium alloy powder, dry it, weigh 250g, add 250g zirconia nano powder, put it into the ball milling tank, add water as dispersant, and mix it by ball milling on the ball mill for 100 hours.
2.1.3 取出混合后的材料,干燥备用。
2.1. 3 take out the mixed materials and dry them for standby.

图2 球磨机
Fig. 2 ball mill
Fig. 2 ball mill
2.2 containers
容器主体是一个内径50mm,壁厚3mm 长500mm的304不锈钢管,
The main body of the vessel is a 304 stainless steel pipe with an inner diameter of 50mm, a wall thickness of 3mm and a length of 500mm,
容器中间设置一根加热套管,套管内径14mm 壁厚3mm长400mm,材质304不锈钢。
A heating sleeve is set in the middle of the vessel, with an inner diameter of 14mm, a wall thickness of 3mm and a length of 400mm, and made of 304 stainless steel.
加热套管中放入加热棒,加热棒直径10mm,长300mm,插入一根K型热电偶,
Put a heating rod with a diameter of 10mm and a length of 300mm into the heating sleeve, and insert a K-type thermocouple,
容器外壁放置一根K型热电偶
A K-type thermocouple is placed on the outer wall of the vessel
容器做保温,保温层用氧化铝纤维棉制作,厚20mm,保温层外做金属护套
The container shall be insulated, and the insulation layer shall be made of alumina fiber cotton, with a thickness of 20mm, and a metal sheath shall be made outside the insulation layer.
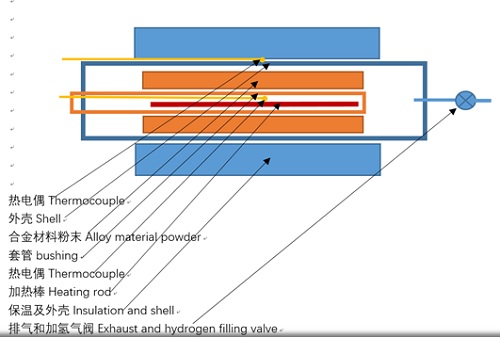
图3 实验容器剖面图
Fig. 3 section of experimental vessel
Fig. 3 section of experimental vessel

图4 实验容器照片
Fig. 4 photo of experimental vessel
Fig. 4 photo of experimental vessel
2.3 量热计
2.3 calorimeter
量热计内腔尺寸200*200*800
Cavity size of calorimeter 200 * 200 * 800
用了200片40*40的热电元件贴附在内腔外表面
200 pieces of 40 * 40 thermoelectric elements are attached to the outer surface of the inner cavity
热电元件外侧是水冷片
Outside the thermoelectric element is a water cooling plate
循环水供水温度25℃
The temperature of circulating water supply is 25 ℃
循环水供水温度波动+-0.01℃
Temperature fluctuation of circulating water supply + - 0.01 ℃
空白试验数据图如下
The blank test data are as follows
2.3 calorimeter
量热计内腔尺寸200*200*800
Cavity size of calorimeter 200 * 200 * 800
用了200片40*40的热电元件贴附在内腔外表面
200 pieces of 40 * 40 thermoelectric elements are attached to the outer surface of the inner cavity
热电元件外侧是水冷片
Outside the thermoelectric element is a water cooling plate
循环水供水温度25℃
The temperature of circulating water supply is 25 ℃
循环水供水温度波动+-0.01℃
Temperature fluctuation of circulating water supply + - 0.01 ℃
空白试验数据图如下
The blank test data are as follows
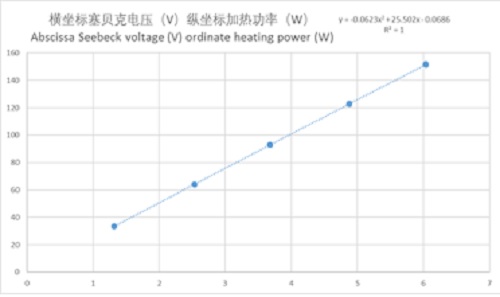
图5量热计空白实验数据图
Fig. 5 blank experimental data of calorimeter
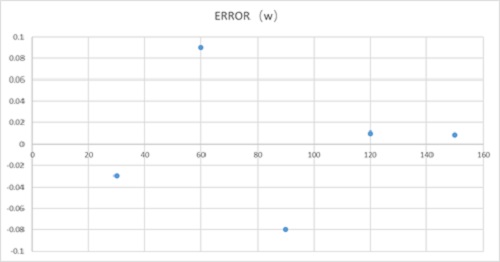
图6、塞贝克量热计误差
Figure 6. Seebeck calorimeter error
Fig. 5 blank experimental data of calorimeter

图6、塞贝克量热计误差
Figure 6. Seebeck calorimeter error
标准误差+-0.1瓦
Standard error + - 0.1W
2.4 实验系统
2.4 experimental system
Standard error + - 0.1W
2.4 实验系统
2.4 experimental system
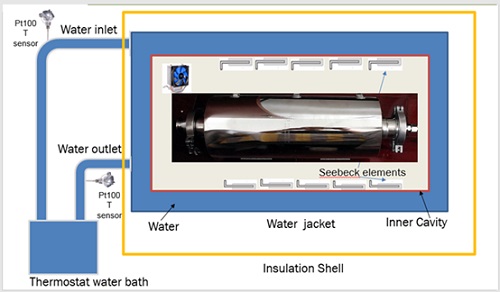
图7 实验装置系统图
Fig. 7 system diagram of experimental device
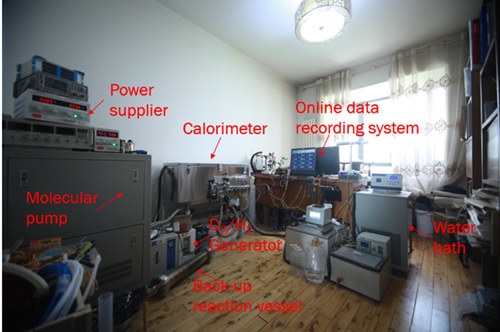
图8 实验装置照片
Fig. 8 photo of experimental device
Fig. 7 system diagram of experimental device

图8 实验装置照片
Fig. 8 photo of experimental device
2.5 实验过程
2.5 experimental process
实验进行了两个多月,过程如下:
The experiment was carried out for more than two months, and the process is as follows:
2.5.1加入合金粉末,将500克锆合金与二氧化锆混合粉末加入容器,其中锆合金重量250克
2.5. 1 add alloy powder, and add 500g of mixed powder of zirconium alloy and zirconia into the container, in which the weight of zirconium alloy is 250g
2.5.2排出气体并注入氢气,氢气压力460kpa,维持氢气压力不变
2.5. 2 discharge the gas and inject hydrogen. The hydrogen pressure is 460kpa and keep the hydrogen pressure unchanged
2.5.3 加热棒开始加热,加热功率维持30瓦60瓦90瓦120瓦150瓦,每个功率维持8-12小时,测量多余热量
2.5. 3. The heating rod starts heating, the heating power is maintained at 30 W, 60 W, 90 W, 120 W and 150 W, each power is maintained for 8-12 hours, and the excess heat is measured
2.5.4 排出气体并注入氢气,氢气压力460kpa,维持氢气压力不变
2.5. 4 discharge the gas and inject hydrogen. The hydrogen pressure is 460kpa and keep the hydrogen pressure unchanged
2.5.5 再次进行加热,加热功率维持30瓦60瓦90瓦120瓦150瓦,每个功率维持8-12小时,测量多余热量
2.5. 5. Heat again. The heating power is maintained at 30 watts, 60 watts, 90 watts, 120 watts and 150 watts. Each power is maintained for 8-12 hours. Measure the excess heat
2.5.6,氧化,排出气体,注入空气,空气压力一个大气压,维持容器450°C,氧化一周时间
2.5. 6. Oxidation, exhaust gas, inject air, the air pressure is one atmospheric pressure, maintain the container at 450 ° C, and oxidize for one week
2.5.7 重复2.5.2步骤
2.5. Seven repeat 2.5 2 steps
3、实验结果
3. Experimental results
3.1 每次氧化后进行氢气实验,可以观察到合金吸氢的现象,并能观察到生成水。
3.1 after each oxidation, the hydrogen absorption of the alloy and the formation of water can be observed.
3.2 每次氧化后进行氢气实验,可以观察到多余热量的升高,第四次和第五次氧化后多余热量升高较大,容器温度变化不大。
3.2 the increase of excess heat can be observed in the hydrogen experiment after each oxidation. After the fourth and fifth oxidation, the excess heat increases greatly, and the container temperature changes little.
3.3 250克铜镍锆合金每次氧化后氢气实验多余热量见下图
3.3 the excess heat of hydrogen test after each oxidation of 250g Cu Ni Zr alloy is shown in the figure below
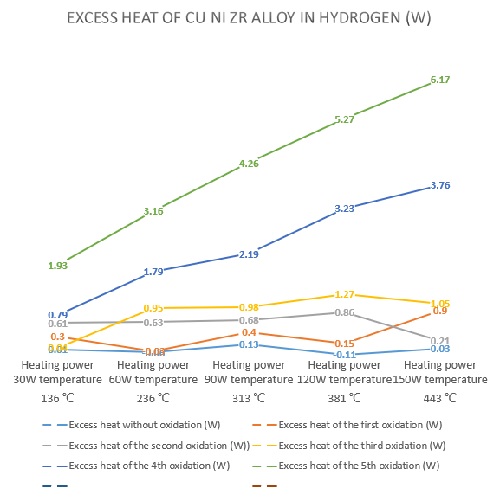
图9、250克铜镍锆合金每次氧化后氢气实验多余热量
Figure 9. Excess heat in hydrogen test after each oxidation of 250g Cu Ni Zr alloy
Figure 9. Excess heat in hydrogen test after each oxidation of 250g Cu Ni Zr alloy
3.4 换算成每公斤锆合金的多余热量,在加热功率150瓦,温度443度,多余热量如下
3.4 the excess heat per kilogram of zirconium alloy is converted into the following when the heating power is 150 watts and the temperature is 443 degrees
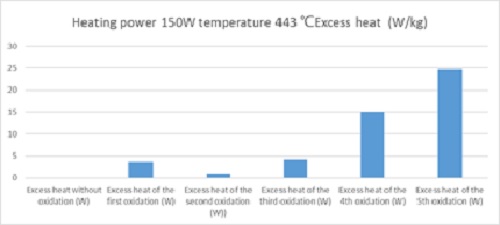
图10、每公斤锆合金的多余热量
Figure 10. Excess heat per kilogram of zirconium alloy
4、结论
4. Conclusion
4.1 铜镍锆合金在多次氧化后在氢气中可观察到多余热量。
4.1 excess heat can be observed in hydrogen after multiple oxidation of copper nickel zirconium alloy.
4.2 每次氧化后充氢实验持续数天,排除了合成水的化学能。
4.2 the hydrogen charging test after each oxidation lasts for several days, excluding the chemical energy of synthetic water.
4.3 日本高桥教授团队的实验现象可以在秋然实验室得到复现
4.3 the experimental phenomenon of Professor Takahashi's team in Japan can be reproduced in Qiuran laboratory
4.4 测量到的多余热量功率高于计量误差5倍以上,可以作为科学证据。
4.4 the excess heat power measured is more than 5 times higher than the measurement error, which can be used as scientific evidence.
4,5 实验测得总多余热量1.7MJ
4,5 the total excess heat measured in the experiment is 1.7mj
鸣谢
Acknowledge
本实验得到高桥教授、李兴中教授、王铁山教授、张武寿博士、陈思博士的指导和帮助。
This experiment was guided and helped by Professor Takahashi, Professor Xingzhong Li, Professor Tieshan Wang, Dr. Wushou Zhang and Dr. Si Chen.
参考资料
Progress in Nano-Metal Hydrogen Energy ICCF23 presentation
Akito Takahashi, Masahiko Hasegawa, Yutaka Mori,Joji Hachisuka, Yuichi Furuyama
4. Conclusion
4.1 铜镍锆合金在多次氧化后在氢气中可观察到多余热量。
4.1 excess heat can be observed in hydrogen after multiple oxidation of copper nickel zirconium alloy.
4.2 每次氧化后充氢实验持续数天,排除了合成水的化学能。
4.2 the hydrogen charging test after each oxidation lasts for several days, excluding the chemical energy of synthetic water.
4.3 日本高桥教授团队的实验现象可以在秋然实验室得到复现
4.3 the experimental phenomenon of Professor Takahashi's team in Japan can be reproduced in Qiuran laboratory
4.4 测量到的多余热量功率高于计量误差5倍以上,可以作为科学证据。
4.4 the excess heat power measured is more than 5 times higher than the measurement error, which can be used as scientific evidence.
4,5 实验测得总多余热量1.7MJ
4,5 the total excess heat measured in the experiment is 1.7mj
鸣谢
Acknowledge
本实验得到高桥教授、李兴中教授、王铁山教授、张武寿博士、陈思博士的指导和帮助。
This experiment was guided and helped by Professor Takahashi, Professor Xingzhong Li, Professor Tieshan Wang, Dr. Wushou Zhang and Dr. Si Chen.
参考资料
Progress in Nano-Metal Hydrogen Energy ICCF23 presentation
Akito Takahashi, Masahiko Hasegawa, Yutaka Mori,Joji Hachisuka, Yuichi Furuyama
分享到:
 收藏
收藏
评论排行
- ·黑光能源公司 BlackLight Power, In...(4294967281)
- ·冷聚变技术可以结束当前的雾霾天气吗?(13)
- ·布里渊能源技术公司的CECR技术简介(9)
- ·Defkalion公司的5千瓦Hyperion冷聚变反应堆(8)
- ·截止2014年国内外冷聚变研究现状(8)
- ·美军宣称已成功将海水直接转化为燃料(8)
- ·关于近期对E-CAT真实性质疑的问题(8)
- ·中核研究院成功复制镍氢冷核聚变装置E-CAT(7)
- ·美预言家预测人类在未来18个月内实现冷...(6)
- ·黑光能源公司宣布改变能源领域游戏规则...(6)
- ·镍氢电能(E-CAT)研究中心落户天津(6)
- ·工业热力公司公告称罗西起诉没有任何价值(6)
- ·空中客车公司对冷核聚变研发感兴趣(5)
- ·1升水能让汽车跑5000公里(4)
- ·冷聚变开拓者:马丁. 弗莱希曼 和 斯...(4)
- ·世界上第一台冷聚变装置E-CAT的研发历程(4)
- ·冷聚变是伪科学吗?(4)
- ·冷聚变将引发环保和新能源技术新的革命(4)
- ·德国EGM公司的水基燃料技术(4)
- ·英国国防部最新报告:冷聚变技术将来会...(4)
