氧化金属氢/氘气氛围异常放热 -西安张航
2021-07-20 22:19:10 来源:冷聚变世界 评论:0 点击:
氧化金属氢/氘气氛围异常放热
Zhang Hang, Chen Si
秋然实验室
Qiuran Laboratory715469127@qq.com
摘要:日本的高桥教授和美国的Storms博士分别发布了文章,他们发现对金属氧化后在氘气或氢气中多余热量会增加,为此进行了实验,发现对金属氧化后在氢气/氘气氛围中多余热量明显增大,验证了高桥教授和Storms博士观察到的现象,这种方法是可以重复复制的。
Abstract: Professor Takahashi of Japan and Dr. Storms of the United States published articles respectively. They found that the excess heat in deuterium or hydrogen would increase after metal oxidation. Therefore, they conducted experiments and found that the excess heat in hydrogen / deuterium atmosphere increased significantly after metal oxidation, which verified the phenomenon observed by Professor Takahashi and Dr. Storms. This method can be replicated repeatedly.
关键词:金属,氧化,氢/氘气,多余热量
Key words: metal, oxidation, hydrogen / deuterium gas, excess heat
1、 高桥教授实验介绍
1、 Introduction of Professor Takahashi's experiment
高桥教授团队用钯镍合金纳米粉末进行实验,进行了两次氧化,每一次氧化后多余热量均有所增加,最高多余热量达到200瓦/kg
Professor Takahashi's team carried out experiments with PD Ni alloy nano powder and carried out two times of oxidation. After each oxidation, the excess heat increased, and the maximum excess heat reached 200 W / kg
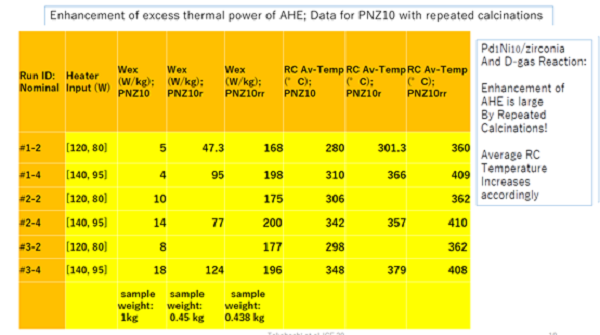
Figure 1, experimental data sheet of Professor Takahashi's team (Literature 1)
2、 Storms博士实验介绍
2、 Introduction to Dr. Storms' experiment
Storms博士用钯粉压成直径约12 mm、厚度约2 mm的饼,重量1.7克,在真空中1000℃煅烧,在氘气氛围中加热用塞贝克量热计测量,量热器精度+-5mW ,没有测得明显的多余热量,接下来在400℃空气中氧化材料,在氘气氛围中加热用塞贝克量热计测量有0.25瓦多余热量的功率,氧化金属可以产生多余热量。
Dr. Storms pressed palladium powder into a cake with a diameter of about 12 mm and a thickness of about 2 mm, weighing 1.7 g, calcined in vacuum at 1000 ℃, heated in deuterium atmosphere, measured by Seebeck calorimeter. The accuracy of the calorimeter was + - 5 mW, and no obvious excess heat was measured. Next, the material was oxidized in air at 400 ℃, heated in deuterium atmosphere, and the power of excess heat was 0.25 watt measured by Seebeck calorimeter, Oxidizing metals can produce excess heat.
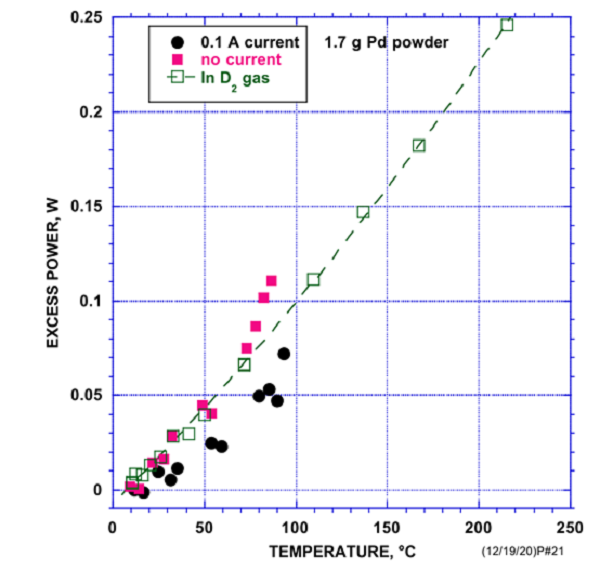
Fig. 2 experimental data of Dr. Storms (ref. 2)
3.1 量热计
3.1 calorimeter
秋然实验室的量热计构造和Storms博士的的基本相同。
The calorimeter structure of qiuran laboratory is basically the same as that of Dr. Storms.
量热计内腔尺寸200*200*800
Cavity size of calorimeter 200 * 200 * 800
用了200片40*40的热电元件贴附在内腔外表面
200 pieces of 40 * 40 thermoelectric elements are attached to the outer surface of the inner cavity
热电元件外侧是水冷片
Outside the thermoelectric element is a water cooling plate
循环水供水温度25℃
The temperature of circulating water supply is 25 ℃
循环水供水温度波动+-0.01℃
Temperature fluctuation of circulating water supply + - 0.01 ℃
空白试验数据图如下
The blank test data are as follows
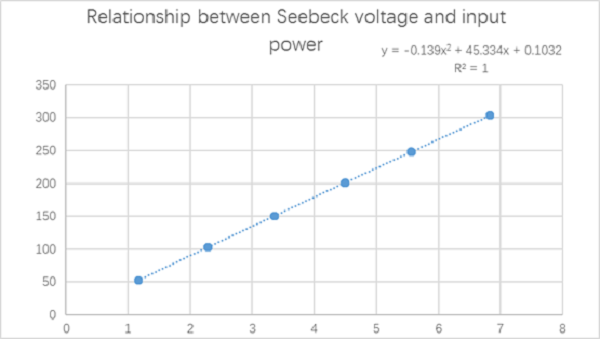
Fig. 3 calorimeter blank experimental data diagram
3.2 实验过程
3.2 experimental process
实验用了一根不锈钢镀钯管,长5米,外径3mm,壁厚0.5mm,内外化学镀钯。镀钯层的厚度1微米。
A stainless steel palladium plated tube, 5 meters long, 3 mm in outer diameter and 0.5 mm in wall thickness, was used in the experiment. The thickness of palladium coating is 1 micron.
实验前材料进行了分析电子显微镜检测,材料表面图像和成分如下:
Before the experiment, the materials were analyzed and examined by electron microscope
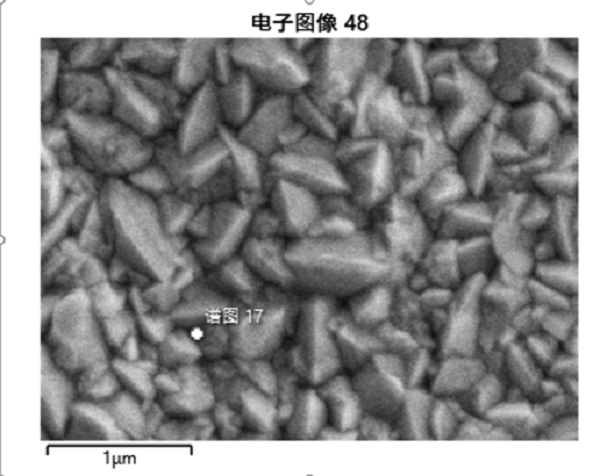
表1 镀钯管实验前元素分析
| 元素 element |
线类型 Line type |
表观浓度 Apparent concentration |
k 比值 K ratio |
Wt% | Wt% Sigma | 标准样品标签 Label of standard sample |
| Pd | L 线系 L-line system |
178.80 | 1.78799 | 100.00 | 0.00 | Pd |
| 总量 total: |
100.00 |
镀钯管一端封闭,绕成螺旋缠绕在加热棒上。
One end of the palladium plated tube is closed and spirally wound on the heating rod.

Figure 5 palladium plated tube wound on heating rod
将镀钯管和加热棒安装到容器中,容器由不锈钢制作,直径100mm。长600mm。
A palladium plated tube and a heating rod are installed in a vessel made of stainless steel with a diameter of 100 mm. 600mm long.

Put the container into the calorimeter,
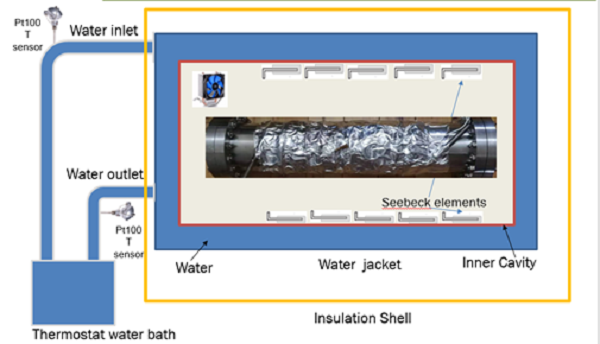
Fig. 7 system diagram of experimental device
The vessel was kept in vacuum, the stainless steel palladium plated tube was filled with hydrogen / deuterium gas, the heating power of the heating rod was kept constant, and the excess heat was measured.
给容器内充入一个大气压的空气,加热棒输入400瓦的功率,在520度温度下维持24小时,然后排出不锈钢镀钯管和容器内的空气。
Fill the container with an atmospheric pressure of air, input 400 watts of power into the heating rod, maintain at 520 ℃ for 24 hours, and then discharge the air from the stainless steel palladium plated tube and the container.
维持加热功率250瓦,容器保持真空,给不锈钢镀钯管内充入氢/氘气,可以观察到在当钯管内氢气压力变化时,有多余热量产生,平衡时也有多余热量产生,这个多余热量功率比氧化前高一些,一次、二次、三次氧化前后氢/氘气实验结果如下
When the heating power is maintained at 250 watts, the vessel is kept in vacuum, and hydrogen / deuterium gas is filled into the stainless steel palladium plated tube, it can be observed that when the hydrogen pressure changes in the palladium tube, there is excess heat generation, and there is also excess heat generation in equilibrium. The excess heat power is higher than that before oxidation. The experimental results of hydrogen / deuterium gas before and after primary, secondary and tertiary oxidation are as follows
2、 秋然实验室实验结果
2、 Experimental results of qiuran Laboratory
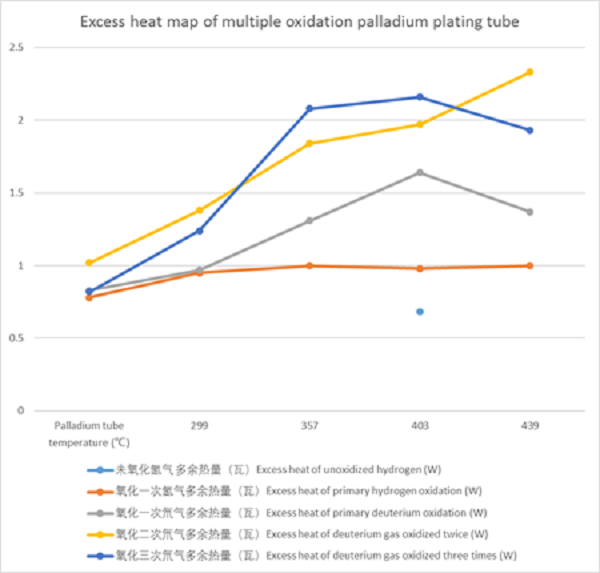
多次氧化后,多余热量有了明显增大,见下表。
After repeated oxidation, the excess heat increased significantly, as shown in the table below.
表2、多次氧化镀钯管氢气/氘气氛围多余热量的功率表
Table 2. Power table of excess heat in hydrogen / deuterium atmosphere of multiple oxidation palladium plating tube
| 钯管温度(℃) Palladium tube temperature (℃) |
未氧化氢气 多余热量(瓦) Excess heat of unoxidized hydrogen (W) |
氧化一次氢气多余热量(瓦)Excess heat of primary hydrogen oxidation (W) | 氧化一次氘气多余热量(瓦)Excess heat of primary deuterium oxidation (W) | 氧化二次氘气多余热量(瓦)Excess heat of deuterium gas oxidized twice (W) | 氧化三次氘气多余热量(瓦)Excess heat of deuterium gas oxidized three times (W) | 加热功率(瓦)Heating power (W) |
| 214 | 0.61 | 0.28 | 0.65 | 0.57 | 50 | |
| 299 | 0.78 | 0.83 | 1.02 | 0.82 | 100 | |
| 357 | 0.95 | 0.97 | 1.38 | 1.24 | 150 | |
| 403 | 1.00 | 1.31 | 1.84 | 2.08 | 200 | |
| 439 | 0.68 | 0.98 | 1.64 | 1.97 | 2.16 | 250 |
| 469 | 1.00 | 1.37 | 2.33 | 1.93 | 300 |
对反应后镀钯管进行了分析电子显微镜检测,从下图可以看出,反应后镀钯管金属表面形态变化较大,呈多孔表面,元素分析也有很大变化,元素中氧氮应该是空气中元素,在氧化时进入材料的,碳可能是污染,钛铁镍应该是基层不锈钢的元素,钯的含量变得极少,其余元素是不锈钢中的杂质。
The palladium plated tube after reaction was analyzed and detected by electron microscope. It can be seen from the figure below that the metal surface of the palladium plated tube after reaction changes greatly, showing a porous surface, and the element analysis also changes greatly. The oxygen and nitrogen in the elements should be elements in the air, which enter into the material during oxidation. Carbon may be pollution. Titanium, iron and nickel should be elements in the base stainless steel, and the content of palladium becomes very small, The other elements are impurities in stainless steel.
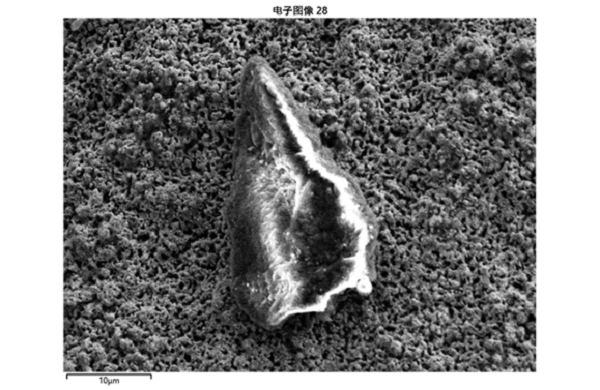
Fig. 10 electron microscope image of surface morphology of palladium plated tube after reaction
表3镀钯管反应后元素分析表
Table 3 element analysis of palladium plated tube after reaction
| 分布图总数谱图 Total number spectrum of distribution map |
||
| 元素 element |
Wt% | Wt% Sigma |
| C | 15.19 | 0.61 |
| N | 0.81 | 0.10 |
| O | 12.71 | 0.21 |
| Na | 0.13 | 0.03 |
| Al | 0.11 | 0.02 |
| Si | 0.24 | 0.03 |
| P | 0.19 | 0.03 |
| K | 0.20 | 0.06 |
| Ti | 10.83 | 1.25 |
| Fe | 32.92 | 0.55 |
| Ni | 25.65 | 0.43 |
| Pd | 1.03 | 0.11 |
| 总量: | 100.00 | |
5、 小结
5、 Summary
5.1 多次氧化可以提高载氢金属多余热量的功率。
5.1 multiple oxidation can increase the excess heat power of hydrogen carrying metal.
5.2 载氢金属多余热量在439摄氏度以下随温度升高而升高
5.2 the excess heat of hydrogen carrying metal increases with the increase of temperature below 439 ℃
5.3 高桥教授和Storms博士的实验结果可以重复和复制。
5.3 the experimental results of Professor Takahashi and Dr. Storms can be repeated and replicated.
5.4 元素分析并未发现有明显的新元素产生
5.4 no new elements were found by element analysis
参考文献
Reference
1、Akito Takahashi, Masahiko Hasegawa, Yutaka Mori, Joji Hachisuka, Yuichi Furuyama Progress in Nano- - Metal Hydrogen Energy ,Talk at The ICCF23 web conference; June 9-11, 2021
2、Edmund Storms, United States ,The Nature of the D+D Fusion Reaction in Palladium and Nickel ,Talk prepared for ICCF-23, June 9-11, 2021
评论排行
- ·黑光能源公司 BlackLight Power, In...(4294967281)
- ·冷聚变技术可以结束当前的雾霾天气吗?(13)
- ·布里渊能源技术公司的CECR技术简介(9)
- ·Defkalion公司的5千瓦Hyperion冷聚变反应堆(8)
- ·截止2014年国内外冷聚变研究现状(8)
- ·美军宣称已成功将海水直接转化为燃料(8)
- ·关于近期对E-CAT真实性质疑的问题(8)
- ·中核研究院成功复制镍氢冷核聚变装置E-CAT(7)
- ·美预言家预测人类在未来18个月内实现冷...(6)
- ·黑光能源公司宣布改变能源领域游戏规则...(6)
- ·镍氢电能(E-CAT)研究中心落户天津(6)
- ·工业热力公司公告称罗西起诉没有任何价值(6)
- ·空中客车公司对冷核聚变研发感兴趣(5)
- ·1升水能让汽车跑5000公里(4)
- ·冷聚变开拓者:马丁. 弗莱希曼 和 斯...(4)
- ·世界上第一台冷聚变装置E-CAT的研发历程(4)
- ·冷聚变是伪科学吗?(4)
- ·冷聚变将引发环保和新能源技术新的革命(4)
- ·德国EGM公司的水基燃料技术(4)
- ·英国国防部最新报告:冷聚变技术将来会...(4)
